|
My graduate research was conducted in the Optical Physics Group at Trent University in Peterborough, Ontario. The long-term goals of the involve the investigation of the varied mechanisms by which atoms can bind together to form molecules, and in particular the formation of highly polar molecules via electron transfer between neutral atoms. As with almost all experimental studies in atomic and molecular physics the main challenge is precise control of light. Lasers can be used for trapping, cooling, exciting, ionizing, associating and dissociating atoms and molecules.
During my time with the group, work was focused constructing optoelectronic measurement and control instrumentation as part of a system for cooling and trapping lithium-7 at sub-mK temperatures in a magneto-optical trap (MOT). Additional work focused on calculating cross-sections for collisional charge transfer, presaging future studies on bound ion-pair systems. The details of my graduate reseach are presented in my masters and doctoral theses.
Figure 1 shows the playground in which the research took place, relating the energy of pairs of isolated atoms to the potential energy curves of the diatomic molecule and molecular ion. Note that each atom pair listed consists of one ground and one excited state atom; because the time between collisions in a MOT is much greater than the excited state lifetime, collisions between two excited atoms rarely take place.[1]
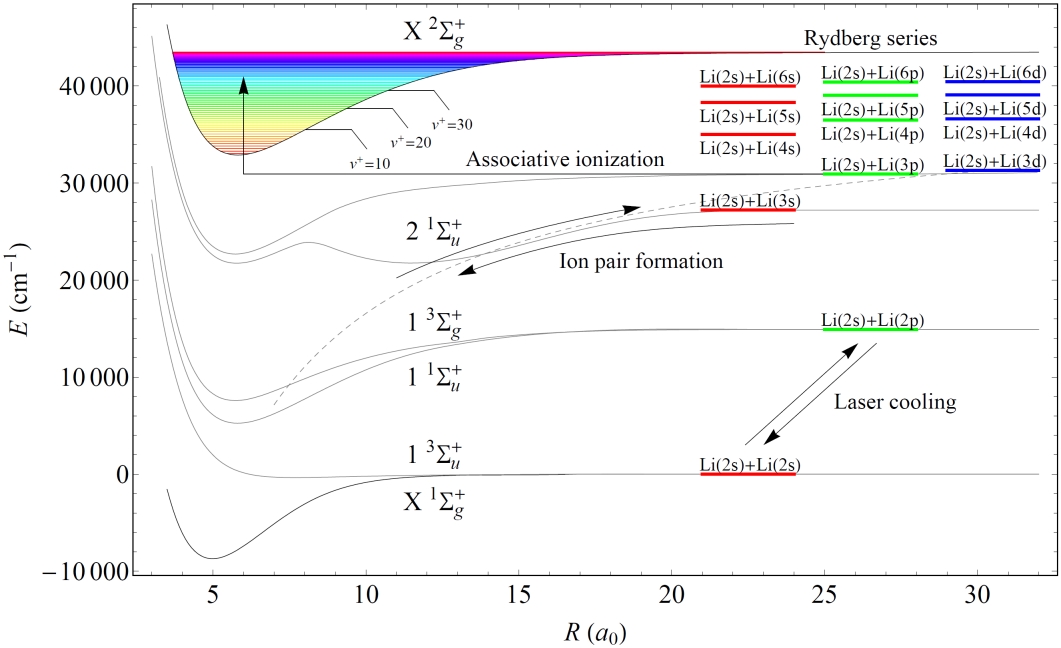
Figure 1: Comparison of the energy levels of a pair of isolated lithium atoms[2] with the ground state potential energy curves of the lithium dimer[3] and its first ionic state[4]. Vibrational levels of the molecular ionic ground state are shown as well as the energy of the Li+-Li- ion pair. Note that the horizontal positions of the atomic levels bear no relation to the internuclear distance axis. |
The long-term goals of the group involve the study of bound ion-pair states commonly known as heavy Rydberg systems.[5] Consisting of a positive and a negative ion bound by the Coulomb force, these systems are in many ways similar to the well understood hydrogen atom with the anion playing the role of the electron. The large reduced mass of the system ensures that dynamic phenomena such as wavepackets occur on long timescales, presenting opportunities for excellent temporal resolution in experiments.
References
^[1] J J Blangé et al., J. Phys. B: At. Mol. Opt. Phys. 30 2789 (1997) ⇒
^[2] C. E. Moore, US Department of Commerce Circular 467
^[3] D.D. Konowalow and J.L. Fish, Chem. Phys. 84 463 (1984) ⇒
^[4] D.D. Konowalow and J.L. Fish, Chem. Phys. Lett. 104 210 (1984) ⇒
^[5] E. Reinhold and W. Ubachs, Molecular Physics 103 1329 (2005) ⇒
Top of Page
|